Abstract
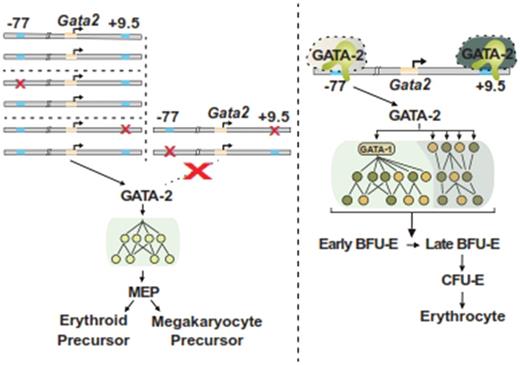
GATA-2 levels must be stringently regulated to ensure normal hematopoiesis, and human GATA-2 mutations cause hematologic disorders. GATA-2-regulated enhancers differentially control Gata2 expression in hematopoietic stem/progenitor cells and are essential for hematopoiesis and embryonic development. Mechanisms underlying how the enhancers control Gata2 expression and GATA-2 instigated genetic networks in a cell-specific manner are not completely understood. Targeted deletion of an intronic Gata2 enhancer 9.5 kb downstream of the transcription start site (+9.5) abrogates HSC genesis in the aorta-gonad-mesonephros (AGM) region (Gao et al., JEM, 2013). By contrast, the -77 kb enhancer (-77) activates transcription in myeloid progenitors, and its deletion impairs progenitor differentiation (Johnson et al., Science Advances, 2015). To dissect relationships between the enhancers, we developed a compound heterozygous (CH) mouse model bearing +9.5 and -77 enhancer mutations on different Gata2 alleles. While the CH embryos were alive at E13.5, nearly all died by E14.5 (p = 3.58 x 10-5). Flow cytometric analyses and embryo confocal imaging demonstrated that CH embryos have modestly reduced HSC numbers in the fetal liver (2.9-fold) and the AGM (41%, p = 7.8 x 10-5), which was comparable to +9.5+/- embryos. Thus, -77 does not genetically interact with +9.5 to control HSC emergence. Flow cytometric analysis revealed that Lin-Sca1-Kit+ myelo-erythroid progenitors were 6.6-fold lower in CH vs. WT embryos (p = 1.8 x 10-11), with the difference involving disproportionate losses of GMP (8.6-fold; p = 3.7 x 10-6) and MEP (379-fold; p = 3.2 x 10-9). By contrast, +9.5+/- fetal livers had 2-fold fewer myeloid progenitors, which involved similar reductions of CMP (2.1-fold; p = 1 x 10-6), GMP (2.6-fold; p = 0.0007) and MEP (1.9-fold; p = 0.002). Consistent with the myelo-erythroid progenitor reductions and MEP depletion, CH fetal livers lacked BFU-E (p < 0.001) and CFU-GEMM (p < 0.001) in a colony assay. These results illustrate a genetic interaction between +9.5 and -77 in progenitors, but not HSCs, and a new paradigm in which both enhancers must reside on a single allele to generate MEPs.
As erythroid precursor cells express GATA-2, we tested whether the -77 deletion impairs erythroid maturation due to a reduction in myelo-erythroid progenitors or due to a cell-autonomous requirement of the enhancer in erythroid precursors. -77-/- E14.5 fetal livers were pale and smaller than WT counterparts, and -77-/- fetal liver cellularity was reduced 7.2-fold (5.3 x 10-4). When liver size was taken into account, there was little difference in the number of E14.5 R1 cells in -77-/- liver vs. WT littermates (p = 0.31). However, -77-/- R2-R5 cells declined sharply (R2, 8.2-fold, p = 0.004; R3, 14-fold, p < 10-5; R4, 9.7-fold, p = 0.002; R5, 14-fold, p = 0.087). The mutant R1 cells were defective in forming BFU-Es and CFU-Es. Analysis of transcriptomes of purified 77-/- and WT R1 cells from E14.5 fetal livers revealed 2805 and 2519 upregulated and downregulated (p < 0.05) genes, respectively, in -77-/- R1 cells. The -77 enhancer conferred GATA-2 expression, which strongly upregulated GATA-1 and therefore a large GATA-1 target gene cohort. A comparison of WT and -77-/- R1 cell transcriptomes with those of early (Tgbfr3low) and late (Tgbfr3high) BFU-Es (Gao et al., Blood, 2016) revealed a -77-/- R1 signature that correlated with the early BFU-E signature (r = 0.73, p < 10-4) and negatively correlated with the late BFU-E signature (r = -0.42, p = 4 x 10-4) differing from WT cells. In addition to GATA-1 target gene alterations, 253 of the -77-activated genes were not GATA-1-regulated in the G1E-ER-GATA-1 system. These genes included Ryk, which encodes a non-canonical Wnt receptor, and had not been studied in erythroid cells. Two Ryk shRNAs significantly decreased BFU-Es and CFU-GMs in lineage-depleted fetal liver cells. Ongoing studies are integrating Ryk function into signaling circuits that control erythroid maturation and analyzing other -77-regulated targets predicted to constitute new regulators of erythroid cell maturation/function. Thus, loss of the -77 enhancer creates multi-faceted defects in erythroid precursors, involving deficiencies of constituents of signaling and transcriptional circuitry required to enable and drive erythroid maturation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal